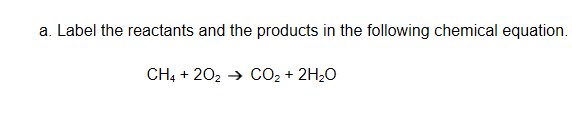Help
CH4 & 2o2 = reactants CO2 & 2H2O = products
perfect
How do an endothermic reaction and an exothermic reaction differ? An endothermic reaction means that it's happening inside and an exothermic reaction means it is happening outside.
that's actually not quite true, exothermic vs endothermic is defined by the energy difference between reactants/products
|dw:1521640953085:dw|
An exothermic reaction is a chemical reaction that releases more energy than it absorbs. An endothermic reaction is a chemical reaction that absorbs more energy than it releases.
exo = releases energy, products have lower energy than reactants endo = absorbs energy, products have higher energy than reactants
yeah, that's about right
egg would be endo?
yes
photosynthesis = ex burning a candle= endo
photosynthesis is actually endothermic (ngl had to look this one up myself) burning something is almost always exothermic
using an instant cold pack- exo using a chemical hand-warmer-exo car engine-endo
combustion (which is involved in the car engine) would be exothermic cold pack - it absorbs heat from the environment so endothermic hand-warmer would be the opposite of a cold pack, it gives off heat so exothermic
In the space below, draw a potential energy diagram for an endothermic reaction and a second potential energy diagram for an exothermic reaction. Give each graph a title, and label the reactants and products on each graph.
This woukld basically just be something like what you posted right?
This?
yes
yes no yes for the first one
|dw:1521641894356:dw|
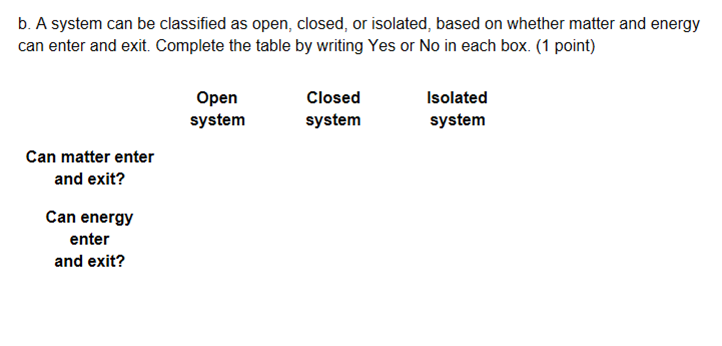
keep in mind we're just talking about matter for the first row
isolated system does not allow either matter or energy so isolated would be no for first row
so yes yes no
or yes no no
YNN
what about row 2?
For energy it needs to be Yes Yes yes
keep in mind an isolated system allows neither energy nor matter
Yes yes no :p
that's correct
Fill in the blanks in the following paragraph to correctly identify the signs associated with heat and work. When a system is heated, heat is _______________ by the system. The amount of heat added is given a _______________ sign. When a system is cooled, heat is _______________ by the system. The amount of heat is given a _______________ sign. If a gas expands, it must push the surrounding atmosphere away. Thus, work is done _______________ the system and is given a _______________ sign. If a gas is compressed, then work is done _______________ the system. This work is given a _______________ sign.
thoughts so far?
um does it have to do with exothermic and endothermic
or expanded or extracted
for the first blank they're probably looking for something like "absorbed"
what about the second blank? positive or negative?
positive
actually neg.
you had it right the first time, heat absorbed gets a + sign
third blank is opposite of absorbed, so released, fourth is the opposite of positive, so negative
for "work done by system" do you remember if the sign is + or -?
positive but work done on the the system is neg
the gas is expanding so we say the work is done (by) the system (since we are looking from the perspective of the system, not the person doing work on it) so 5th blank is "by" and 6th is positive
last two blanks are just the opposite of those two blanks, so "on" the system and negative sign
an then work done "on:
Yup that's what I was going to say
What is the change in internal energy if 50 J of heat are released from a system, and the system does 80 J of work on its surroundings?
the sign convention can be tricky, but if heat is being released what's the sign on heat, and if the system is doing work what's the sign on work?
the sign convention can be tricky, but if heat is being released what's the sign on heat= pos if the system is doing work what's the sign on work= neg
heat is being released from the system, so from the system's perspective it's losing heat work done (by) system is positive so Q = neg, Work = positive so if U = Q - W, U = ?
-50-80?
awesome, so -130J
a. Fill in the blanks in the following paragraph to correctly identify four principles of the second law of thermodynamics. Heat naturally flows from an object that has a _______________ temperature to an object that has a _______________ temperature. Heat can be made to flow in the reverse direction if _______________ is done. A machine can never have an efficiency of _______________. This means that heat energy can never be fully converted into _______________ energy.
high temp to low temp?
yes for the third blank, any ideas? what has to be done to make heat flow from low to high temp?
work
awesome any ideas for the efficiency question?
um energy?
efficiency is usually given as a percentage, what percentage efficiency would be impossible according to the laws of thermodynamics?
100
good, so 100 percent goes into the blank the last blank I'm actually not 100% sure but a good hunch would be "mechanical" :S
oh okay, i was leaning towards work but I am just guessing so I will do with mechanical
yeah I just double checked and it seems like it would be mechanical since work isn't classified as a type of energy
perfect
Describe entropy. What is the natural tendency of the entropy of a system? Entropy is an important concept in physics that uses microscopic aspects to describe the thermodynamics of the systems of molecules. The natural tendency of the entropy of a system is to increase over time.
good start, probably should mention that entropy is the number of states/configurations a system can have
Entropy is an important concept in physics that uses microscopic aspects to describe the thermodynamics of the systems of molecules. It is also the number of the variations of states and/or configurations a system can have. The natural tendency of the entropy of a system is to increase over time.
good
How does a heat engine differ from a heat pump?
Heat Engine is the system that converts the Heat energy into mechanical work. while Heat pump converts the work into heat. Heat Engine is the system that converts the Heat energy into mechanical work.
good (make sure you delete that last sentence since you repeated yourself)
Im gonna open a new thread this is getting long
sure
Join our real-time social learning platform and learn together with your friends!