How many atoms are in 12.53g of Fe(NO3)3
grams to moles first then atoms right
yeah
when it says atoms does it give a specific type of atom?
I think it's a little strange that they're asking for atoms not molecules
okay so I would find the molar mass of Fe(NO3)3 divide it by weight and then multiply by avogadros'
mass/molar mass ---> moles so 12.53/whatever molar mass
Nope it just says how mant atoms are in 12.53g of Fe(NO3)3
0.05199
I calculated the molar mass of Fe(NO3)3
and did 12.53/241
yeah that should be right (for accuracy I would leave a few more digits on 241, it would be 241.8597g/mol with less rounding) 12.53/241.8597 = 0.05180 moles then from there, multiplying by avogadro's will give you the # of molecules
since it asks for atoms, not molecules, I ~think~ you would also multiply by 13 since there are 13 atoms in every Fe(NO3)3 molecule but not 100% sure on this Fe(NO3)3
multiply by 13 before or after
multiplication is commutative so the order doesn't really matter
like before multiply by avogadros or after
true.
4.055 x x 10^24 atoms
yeah that's what i got too if you have enough time i can double check really quick w/ someone, if not we can move on
Yeah I have time its a hw sheet
we can move on to the next problem, I'll let you know if something needs to be re-done
Okay
How many grams are in 3.30 mol of W? so 183.85 * 3.30 = 606.705 g
yeah that's what i got too, just after sig figs it's 607g
How many mol are in 1.64 x 10^23 atoms of Pb so
atoms to moles you divide
so would I do 1.64 x 10^23 / 207.19?
close, atoms to moles ---> divide by avogadro's not the molar mass
since molar mass = g/mole you would either have to start with grams or want to end up with grams, that's not the case here
0.272 mol?
yeah that's what i got too
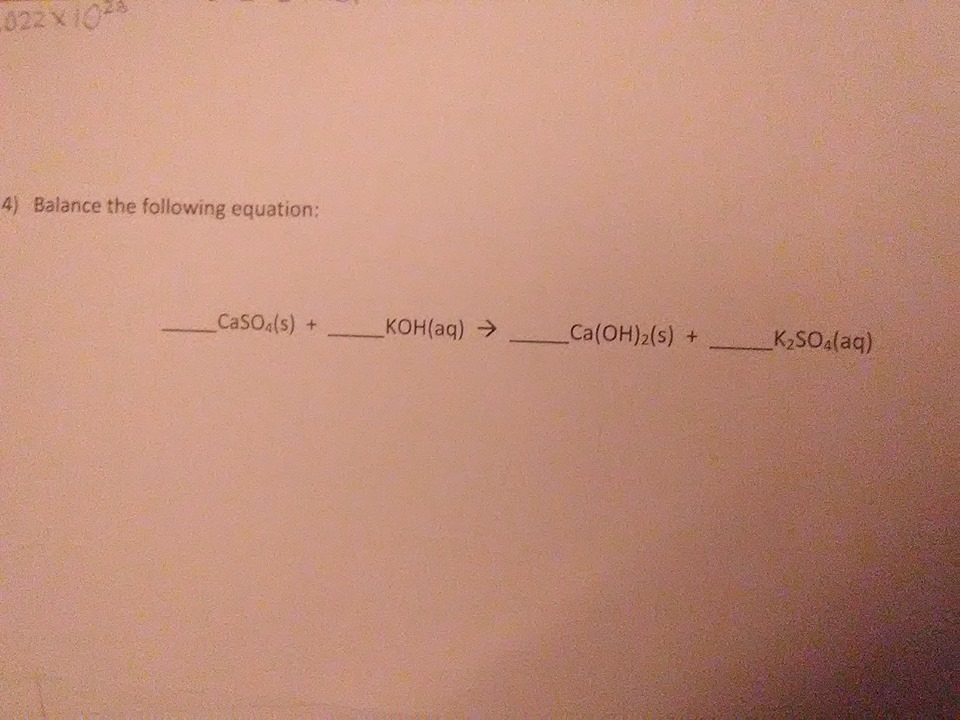
COmpletely stumped on this if you could just go over how to balance
oh well this involves some trial and error, but you want to write whole # coefficients such that every element is balanced on both sides (ex. both sides should have the same # of potassium atoms, oxygen atoms, sulfur... etc.)
Well I know that on both sides there is a common divisor
as a general rule you start with the most long/complex-looking compound and try to balance its elements for example, on your question, I would start with K2SO4. since the right hand side has 2 potassium atoms (K2SO4) and the left side only has 1 (KOH) I put a 2 in front of KOH to balance out the potassiums
coincidentally for this question that completes the balancing (after you write a 2 in front of KOH all the other atoms will be balanced too)
so every other compound just gets a 1 coefficient since we didn't really change those
OH so start from the longest and go from there?
yeah, that's a good rule-of-thumb
OKay sounds good. That is all the work I had for today =) Thank you so much . I appreciate it
awesome I double checked w/ a friend for that first question and they said it was correct
Great. Thanks
Great. Thanks
Join our real-time social learning platform and learn together with your friends!