GUYS ITS DUE IN 2 HOURS AND I CANT DO HALF REACTIONS HELLLLP PLEASE ap chem a) Identify the oxidation and reduction half-reactions for the equation below and describe the number of electrons that are involved in the reaction. 2Ag+(aq) + Pb(s) → 2Ag(s) + Pb2+(aq) b) Explain the reaction that would occur in a galvanic cell between solutions of Al3+ and Pb2+. Identify the oxidation and reduction reactions and the location of each reaction. Then, use the standard reduction potential tables to determine the cell potential.
To analyze this redox reaction, let's break it down into its oxidation and reduction half-reactions and determine the number of electrons involved. 1. Identifying the half-reactions: Reduction half-reaction: Ag+ (aq) + e- → Ag(s) Oxidation half-reaction: Pb(s) → Pb2+ (aq) + 2e- 2. Number of electrons involved: In the reduction half-reaction: - Each Ag+ ion gains one electron to become Ag(s) - There are two Ag+ ions in the overall equation - Therefore, 2 electrons are gained in total In the oxidation half-reaction: - Each Pb atom loses two electrons to become Pb2+ - There is one Pb atom in the overall equation - Therefore, 2 electrons are lost in total The number of electrons lost in the oxidation half-reaction equals the number of electrons gained in the reduction half-reaction, which is 2 electrons. In summary: - Reduction: 2Ag+ (aq) + 2e- → 2Ag(s) - Oxidation: Pb(s) → Pb2+ (aq) + 2e- - Total number of electrons transferred: 2 electrons
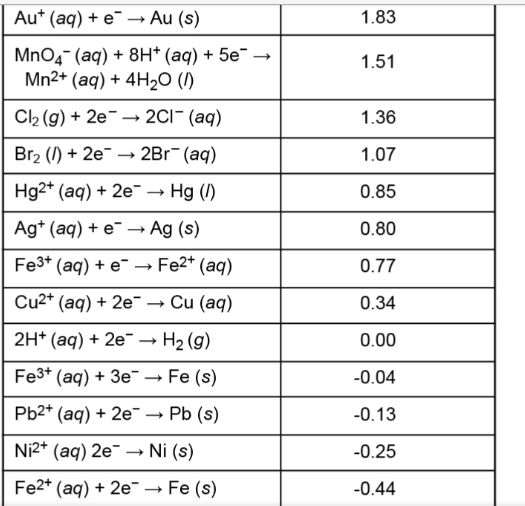
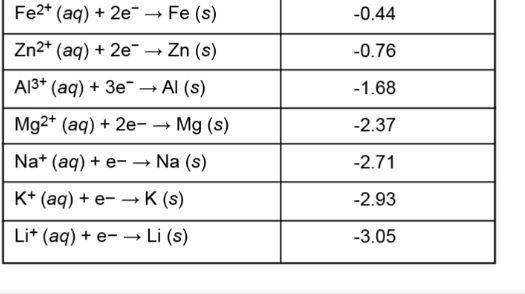
In a galvanic cell between solutions of Al3+ and Pb2+, the following reaction would occur: 2Al(s) + 3Pb2+(aq) → 2Al3+(aq) + 3Pb(s) The half-reactions and their locations are: Oxidation (occurs at the anode): Al(s) → Al3+(aq) + 3e- Reduction (occurs at the cathode): Pb2+(aq) + 2e- → Pb(s) To determine the cell potential, we need to use the standard reduction potentials: Al3+(aq) + 3e- → Al(s) E° = -1.66 V Pb2+(aq) + 2e- → Pb(s) E° = -0.13 V The cell potential (E°cell) is calculated by subtracting the oxidation potential from the reduction potential: E°cell = E°reduction - E°oxidation E°cell = E°(Pb2+/Pb) - E°(Al3+/Al) E°cell = (-0.13 V) - (-1.66 V) = 1.53 V Therefore, the standard cell potential for this galvanic cell is 1.53 V[1][2].
TYSM!
Join our real-time social learning platform and learn together with your friends!