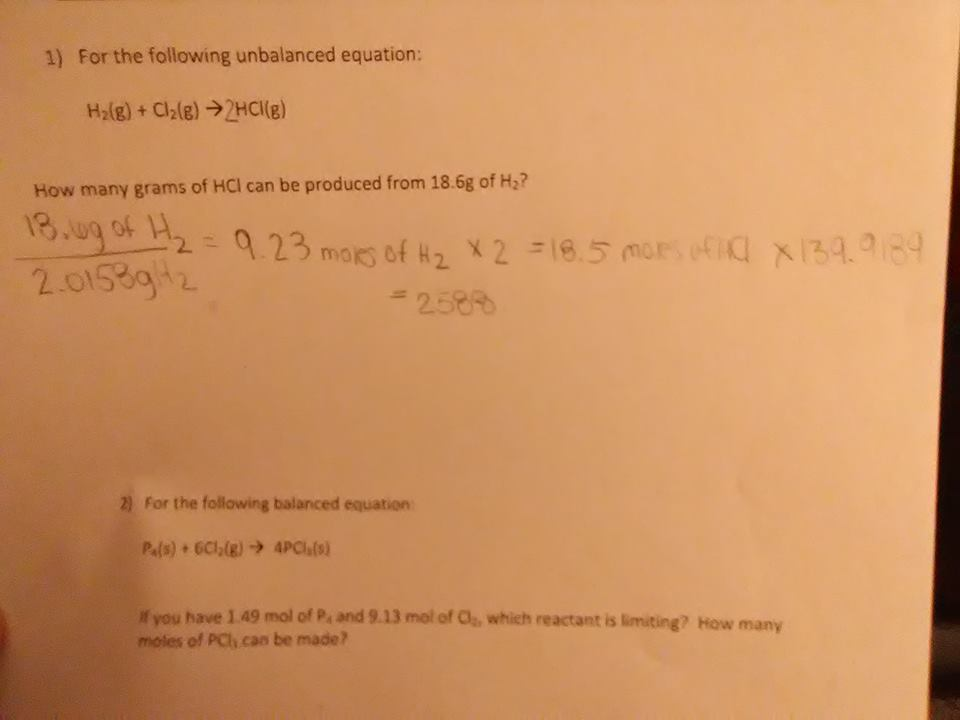For the following unbalanced equation: H2(g) + CI2(g) --> HCI(g) How many grams of HCI can be produced from 18.6g of H2
So, 18.6 grams of H2 equates to 9.3 moles of H2 and 18.6 moles of H atoms. I think this means that you can make 18.6 moles of HCl, assuming you have enough Cl. Now, since HCl has a molar mass of 36.46 g/mol, 18.6 moles of HCl would have a mass of 678.16 grams.
@Vocaloid can you explain this? I'm still alittle confused
the above method works, it's just a little roundabout H2(g) + CI2(g) --> HCI(g) personally i'd start by balancing the equation (notice that putting a 2 in front of HCl balances the equation) H2(g) + CI2(g) --> 2HCI(g)
then it's just stoich 18.6g of H2 ---> divide by the molar mass of H2 ---> that gives you moles of H2 --> multiplying by 2 gives moles of HCl since 2 moles of HCl are produced for each 1 mole of H2 --> multiply by molar mass of HCl to get grams
2588
hm, nah, smokey's original calculation of 678-ish should be right may I see your calculations? I may be able to pinpoint what went wrong
molar mass of Hcl is not 139-whatever, it's 36.46
oh okay
18.6g/2.0158 * 2 * 36.46 should give your your sol'n
672.84
good, they only give us 3 sig figs so round that to 673 g
This would be the same concept right
sort of you need to calculate a) how many moles of PCl3 you can make with 1.49 mol P4 and b) how many moles of PCl3 you can make with 9.13 mol Cl2 then the smaller quantity from a) vs b) = your limiting reagent
pay attention to the mole ratios given by the equation
so I would divide for the first part
you need to set up a conversion factor between moles P4 and moles PCl3 based on the chemical equation
CI2 is limiting
I know that :S
Would i do P4/4PCi
|dw:1539912010305:dw|
you want to set up the conversion factor so that P4 cancels out.
ok
6?
6 moles of PCl? yeah (round to 5.96 as we are given three sig figs)
then repeat the process with Cl
54.8
|dw:1539912681469:dw|
6
to be more specific, 6.09 (remember, 3 sig figs)
so, you end up with less product using P4 so P4 is your limiting reagent, and from the calculations earlier, we know that 1.49 mol P4 makes 5.96 mol PCl3 ---> your solution
Join our real-time social learning platform and learn together with your friends!