AP Chem Practice Helllp please. I have no idea where to start! Replace the reactant specified in each of the following compounds with another element based on periodic properties of the periodic table. Justify each answer using the electron configurations of the elements. a. Replace Al in Al + O2 → Al2O3. b. Replace Br in Be + Br2 → BeBr2.
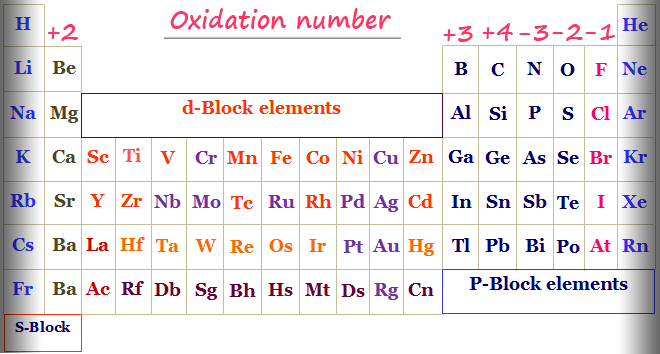
okay, so first do u have an oxidation chart
because u'll need it for this
lemme check
and are u confused on where the 3 came from on Al2O3
because thats the oxidation number of Al they swapped electrons and also electrons cn have a different number of oxidation number so don't forget that
so i think the question is asking u to swap the Al and Br with another element that as the same amount of oxidation number
ohhhh ok
so anything on their collumn can be put for their answer
what do u think?
tbh it make sense to me but I don't know enough to confirm it. Thanks for the help!!!
furthermore, I tried to replace them with ones from the same group (reactive nonmetals for one, post transition metals for the other)
Join our real-time social learning platform and learn together with your friends!