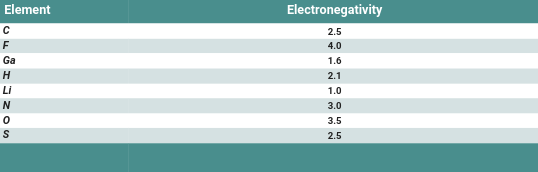
Use the table of electronegativities to rank the following bonds according to polarity. Justify your answer. C—C N—H Li—F H—S Ga—O Low key I am soooo confused on how to do this, can someone explain the process (I put the table below btw)
Electronegativity is directly related to polarity, we can look at the electronegativity for one atom, and find the difference with the other via subtraction. The higher the difference, the higher the polarity. The opposite is also true, lower electronegativity difference means low polarity. For example, a C-C bopnd is 2.5 electronegativity to 2.5, which would be a difference of 0, meaning it is a completely non-polar bond. You'll learn this later on in biology especially, but lipids (fats) are very nonpolar because they contain a lot of hydrocarbon bonds (C and H bonds) which have very low electronegativity difference. I'll do one more example and let you do the rest. N-H bond, is 3.0 to 2.1, which is a 0.9 difference, which is somewhat polar, more polar than a C-C bond Essentially use the table to plug in the electronegativities, and then subtract them to find the difference. The biggest number is the most polar, while the least number is the least polar. Remember that negative electronegativity difference does not exist, and all values should be made positive for an accurate comparison. Let me know if you are confused on anything and I'll gladly help some more!
TYSM!
can I give medal? I'm not sure how it works
ok I figured it out! thanks again!
Join our real-time social learning platform and learn together with your friends!