Help
@Vocaloid
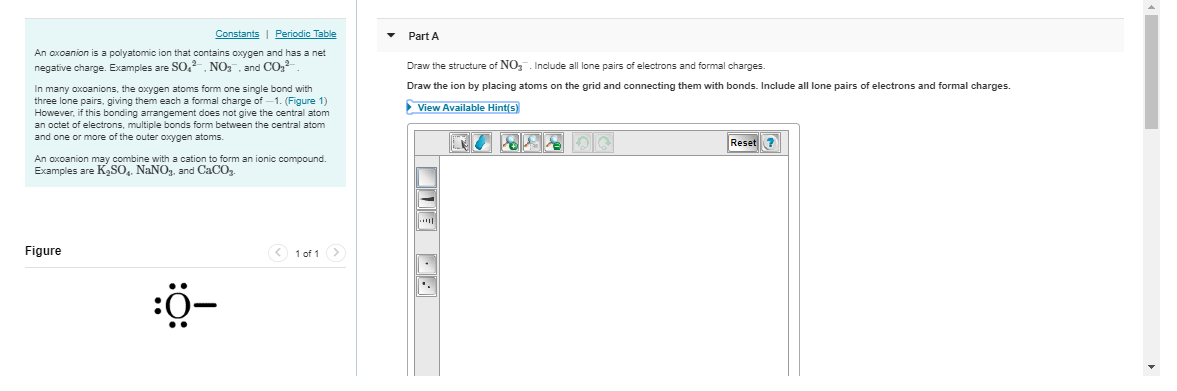
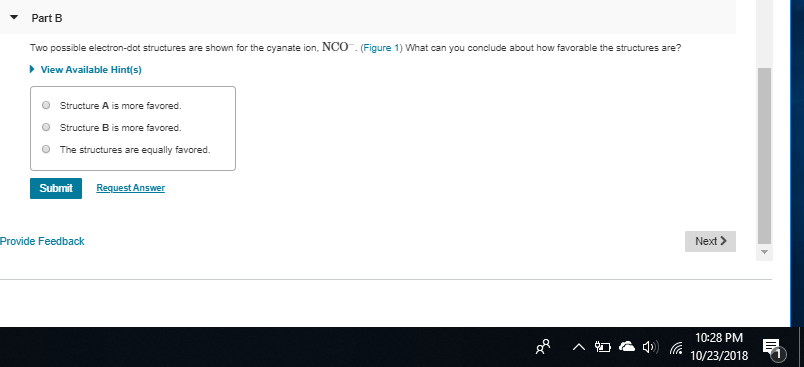
you'd add up the valence electrons as usual, add 1 more since we have a -1 charge, divide by 2 to get the total # of e' pairs, and arrange them following lewis structure rules
give it a shot and i'll step in if you're getting stuck or want a solution check
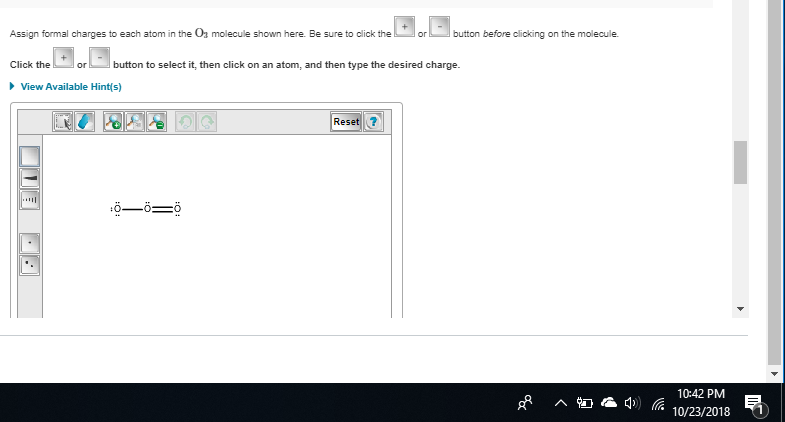
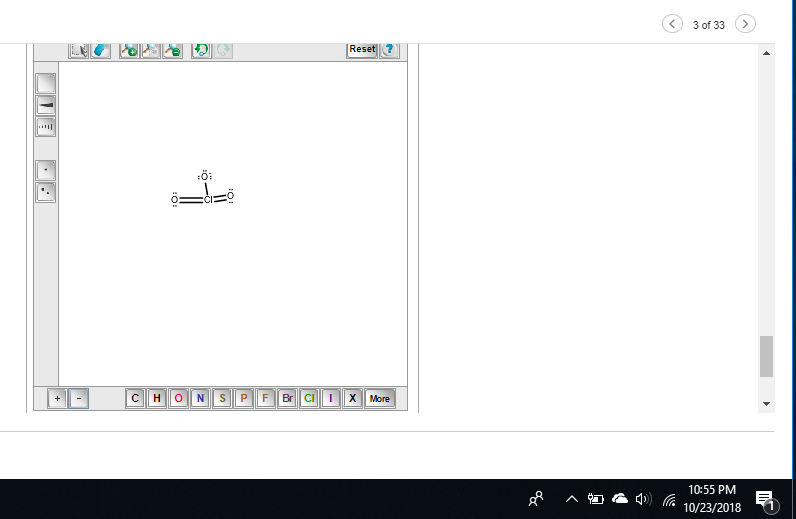
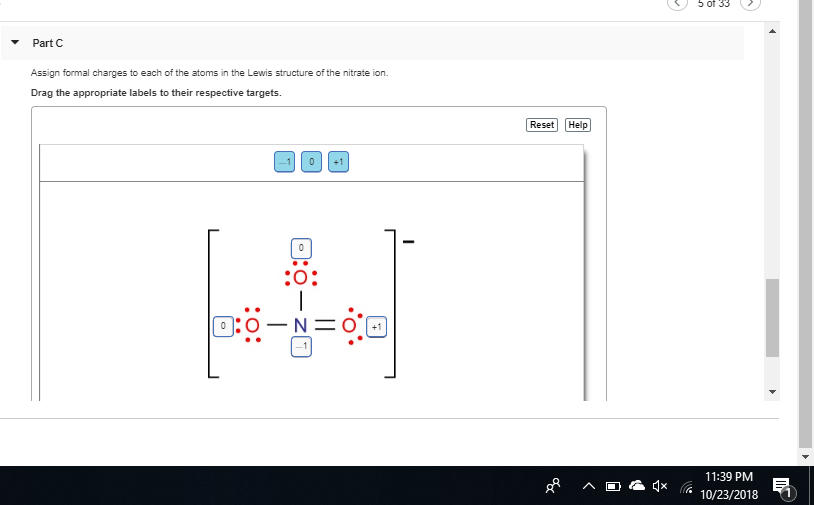
O added a -1 charge to the two o's wiTH A single bond
I added *
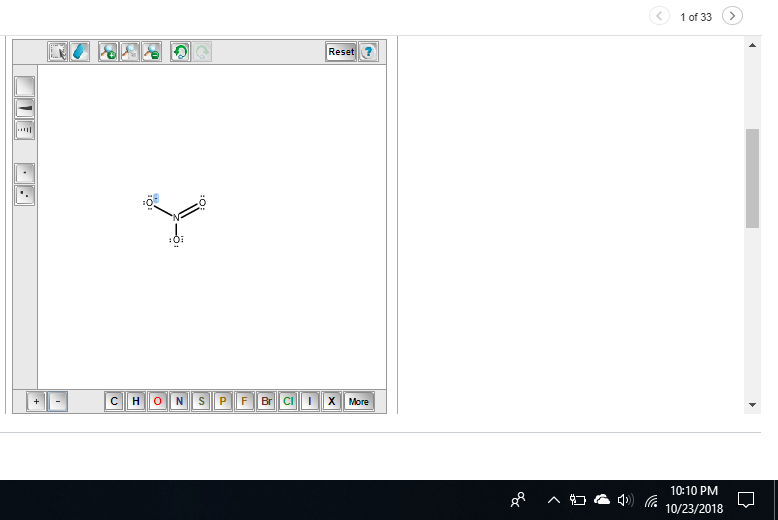
almost nitrogen has a formal charge of 1 here since it normally has 5 valence electrons but only 4 bonds here and 0 lone pairs (5 - 4 - 0) = +1 so you need to add a + to the central nitrogen
if you want to double-check yourself in the future, since NO3- has an overall charge of -1, the formal charges need to sum up to -1 if we only put two negative charges on the oxygens, that's an overall formal charge of -2, not -1, so we know we need to add a +1 somewhere
FOr this I know the overall charge is -2 but I don't know where to put that
like at the top
notice the formal charges on the single-bond oxygen oxygen has 6 valence electrons. in its current state it has 6 lone electrons and 1 bond, so its formal charge is 6 - 6 - 1 = -1 so each of the single-bonded oxygens gets a -1 formal charge making the overall formal charge -2
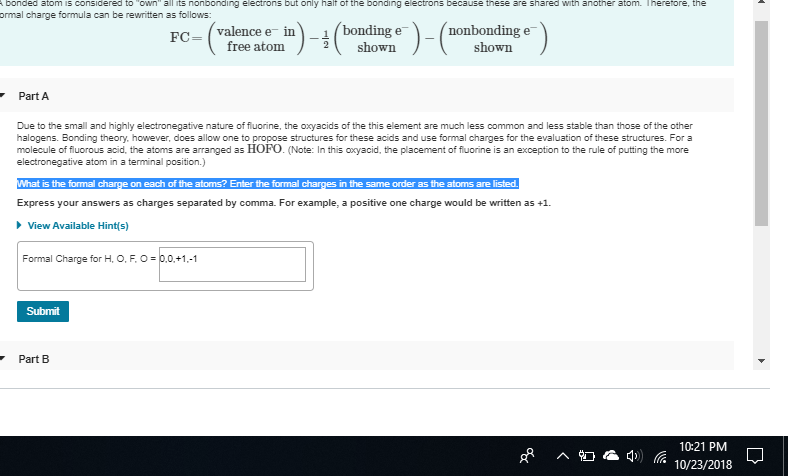
What is the formal charge on each of the atoms? Enter the formal charges in the same order as the atoms are listed. of HOFO
may I see your lewis structure? as far as I know I don't think any of the atoms have formal charges
|dw:1540348011551:dw|
H-O-F-O
ah, if they want all single bonds then your solution would be correct
B
depends on what the structures are
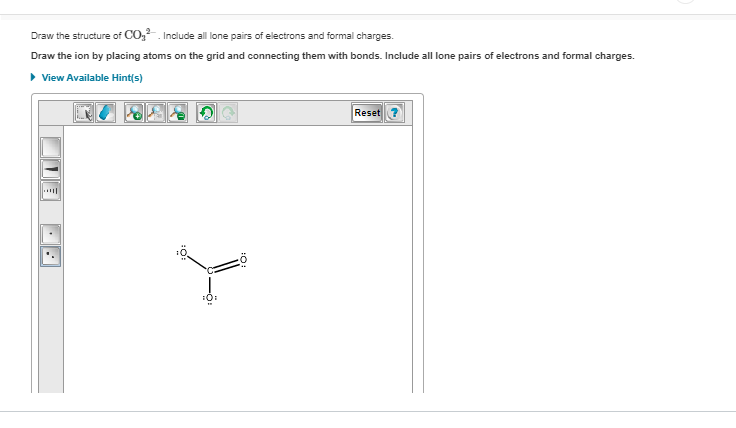
whatever structure puts more electrons on the more EN atom, in this case, oxygen, would be the right structure
These are the hints so I was going off of this
That it is better
i still need to see what the structures are
I got it lol thanks though
and two dots on the p which I forgot
good
so to get the minus I would put it on the single oxygen right
yup
SO this would be a plus on the middle o
yes, any other formal charges you can see?
minus on the single o
good
minus on the single o
yeah i messed up before it's supposed to have 2 double bonds not 1
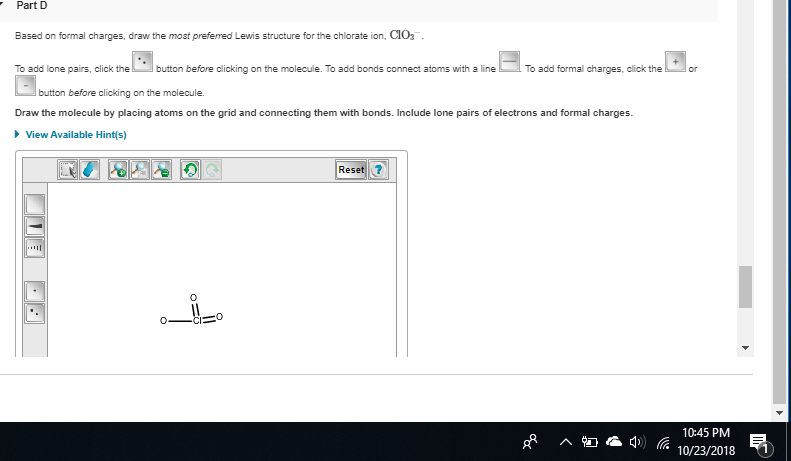
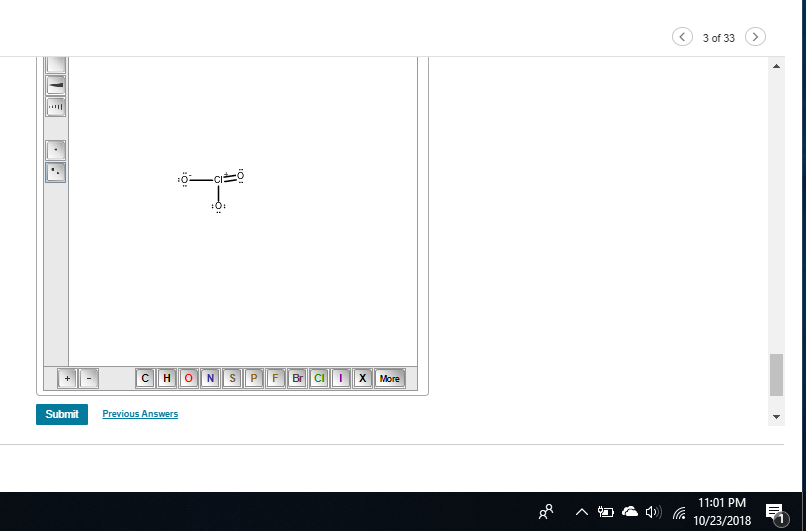
otherwise, good, then add the formal charges on oxygen and chlorine
so minus on the single o
yeah that's it
chlorine shouldn't have one I messed up my structure before whoops
It says its incorrect and I need to check the electrons
Did you overload an octet?
Because I see 5 bonds on Chlorine
chlorine has an expanded octet and should theoretically be able to take more than 4 bonds but try removing one of the double bonds to give chlorine a +1 FC
and putting two dots on the one that I make a single bond right
Well I learned something new about Lewis structures today, thanks Voca
^^'
Okay I'm leaving now so you guys can discuss lol
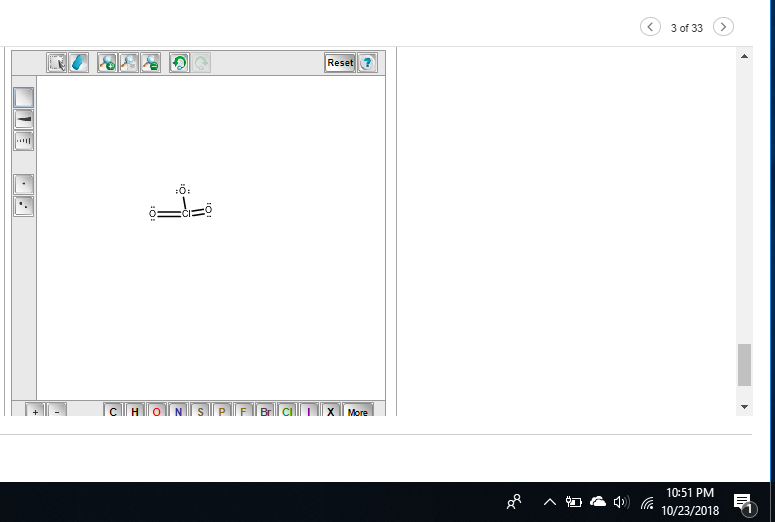
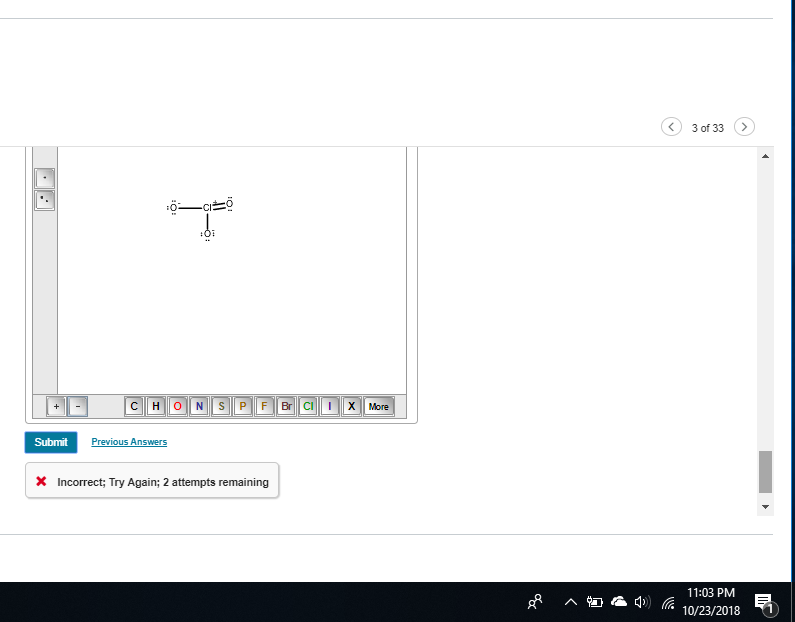
yeah, Cl with 2 single bonds to O, 1 double bond to oxygen, formal charge -1 on the single bond oxygen, and +1 on the chlorine
Nope :/ still wrong
you need -1 on both single bonded oxygens
Still wrong
huh.
do I add something to the double bonded one maybe
no, that one has formal charge 0 so it shouldn't need anything.
oh the one you have right now needs an electron pair on chlorine
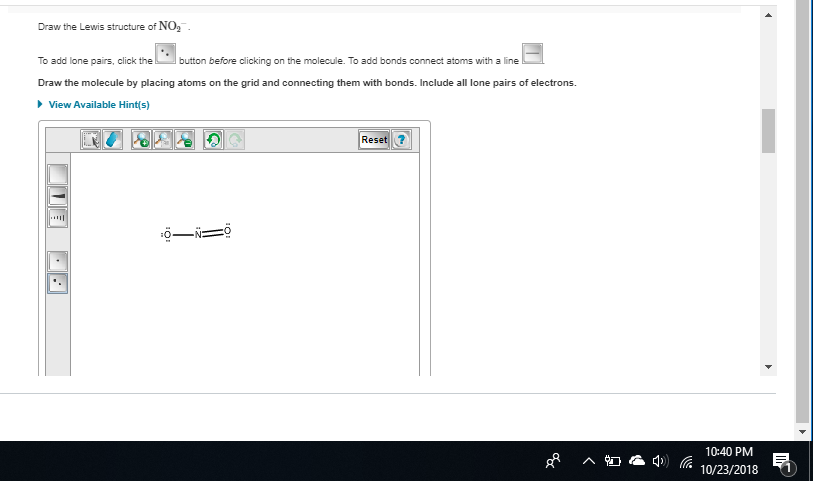
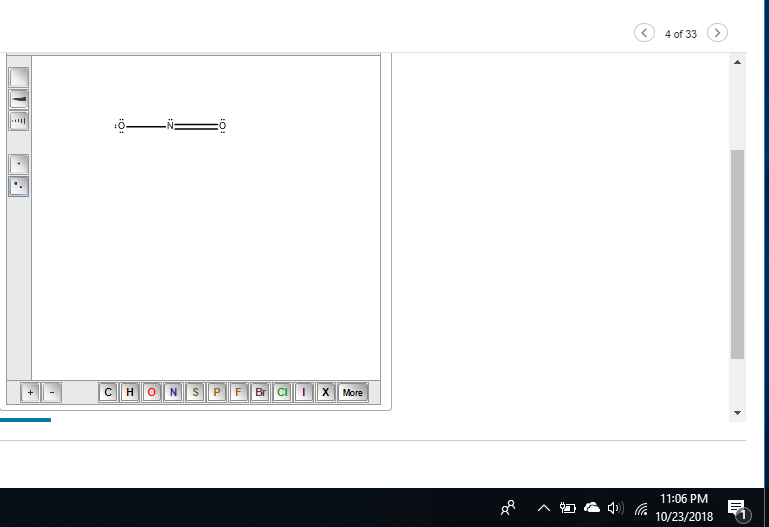
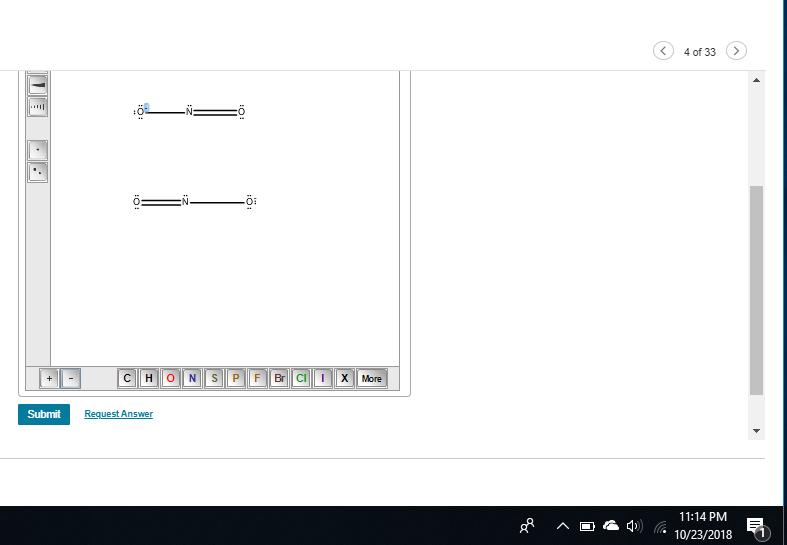
Draw the Lewis structures for the NO2− ion. Include all resonance structures.
what does resonance mean
this is something to review but it means you need to draw all possible structures of NO2-
|dw:1540350690550:dw| there, this is a better image
these are both resonance structures because they're the same compound just with different distributions of charge
okay so is the structure I did now okay if it is I can make the second one to it
yeah just don't forget formal charges if they ask for them
yeah that should be it, lmk how it goes
it was right!
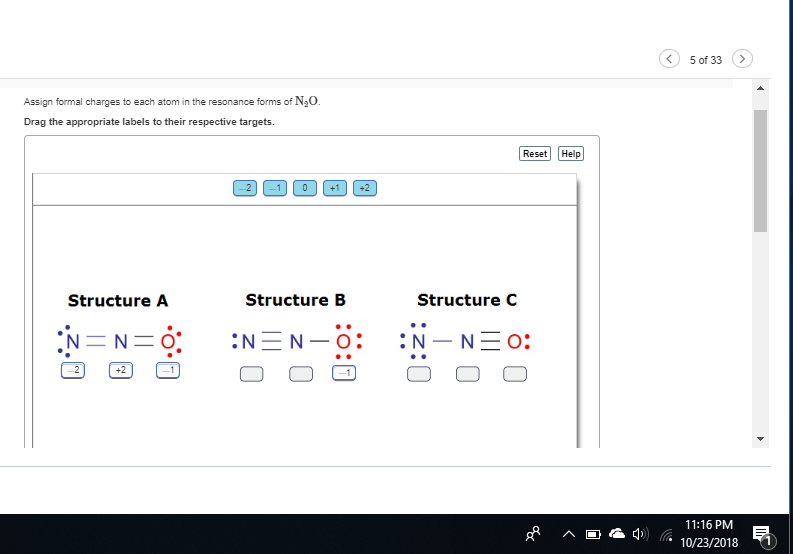
would triple bonds be +2?
formula for formal charge: valence electrons - (number of bonds) - (number of lone pairs)
try to apply it to the first nitrogen
so 5 ve in n
6 in o
yes, keep going
16
16?? where is this coming from
the n20
you don't have to add up the whole molecule; consider each atom individually
oh okay then 10
for the first nitrogen what is valence electrons - (number of bonds) - (number of lone pairs)
10-2-0=8
nitrogen has 5 valence electrons the nitrogen has 2 bonds and 4 lone pair electrons so 5 - 2 - 4
-1
yes try to apply this logic to the other atoms
for clarity's sake *** valence electrons - (number of bonds) - (number of lone pair electrons) ***
okay so 5-2-4 again ? :S
for the central nitrogen you have valence electrons = 5 4 bonds 0 lone pair electrons.
1
good try to see what you get for oxygen
6-4?
-0
you also have 2 bonds
6-2-0=4
6 valence electrons 2 bonds 4 lone electrons
0
good
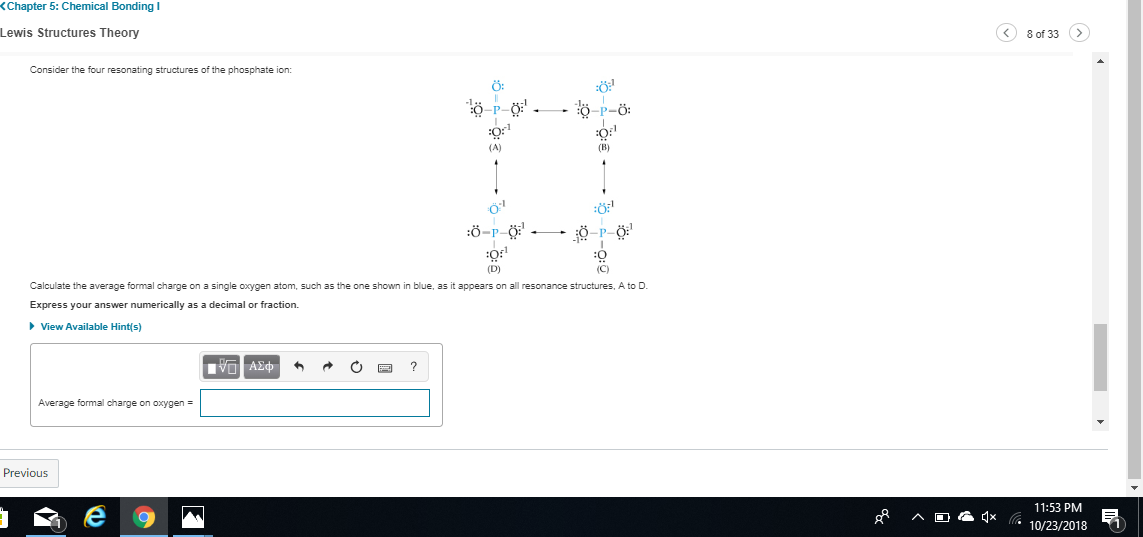
so -1 , +1 , 0 for structure A see what you get for the others
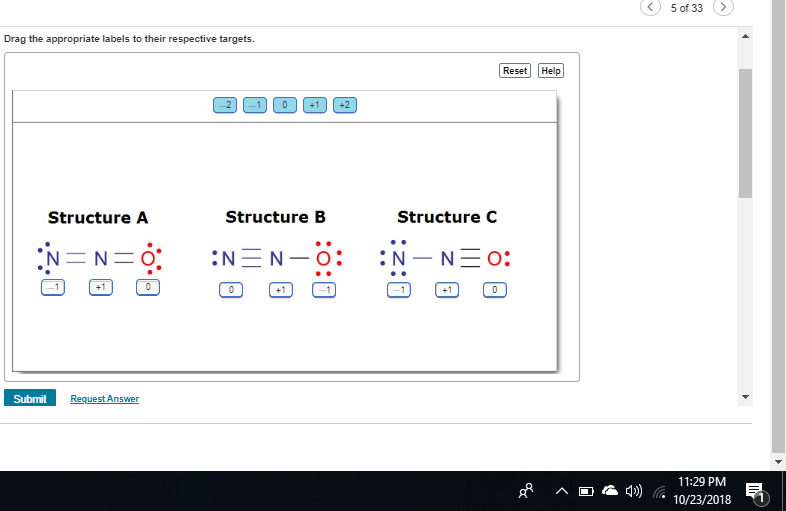
check C again
0 and -1 switched
first nitrogen 5 valence 1 bond 6 lone electrons
-2
0
for the next
good central nitrogen is +1 as you stated check the oxygen now
+1
good so C = -2, +1 +1
A
not quite nitrogen is less electronegative so it will want a higher formal charge oxygen will want a lower formal charge
c
No B
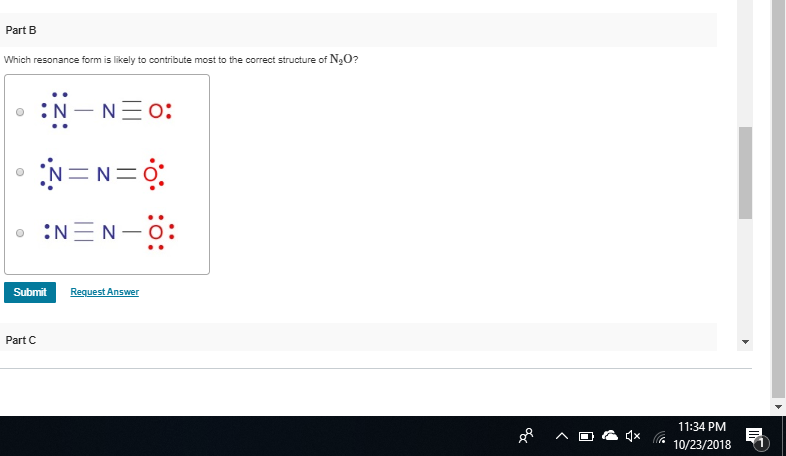
on structure B, you have -1 +1 0 structure C = 0, +1, -1 C puts the +1 on the nitrogen and the -1 on the oxygen which is preferable to structure B
yeah try re-doing them from the top for each of the single bond O's you have 6 valence electrons 6 lone electrons 1 bond
double bond 0 has +1and n has 0?
single bond O's: 6 - 6 - 1 double bond O's: 6 valence - 2 bonds - 4 lone electrons nitrogen: 5 valence electrons - 1 bond - 0 lone electrons
nitrogen: 5 valence electrons - 1 bond - 0 lone electrons is nt an option for 4
*4 bonds
5 valence electrons - 4 bond - 0 lone electrons
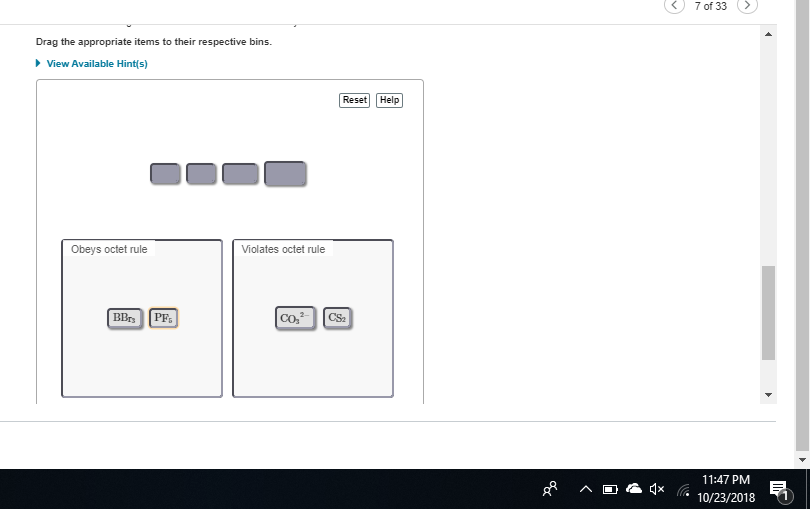
you need to swap them carbon always follows octet; boron and phosphorus violate the octet rule (boron only makes 3 bonds whereas phosphorus can make more than 4)
co or cs
co right
both of them have carbon as the central atom and thus follow the octet rule
uh as far as I know you'd just add up the oxygen formal charges and divide by 4
Join our real-time social learning platform and learn together with your friends!