Ask
your own question, for FREE!
Chemistry
22 Online
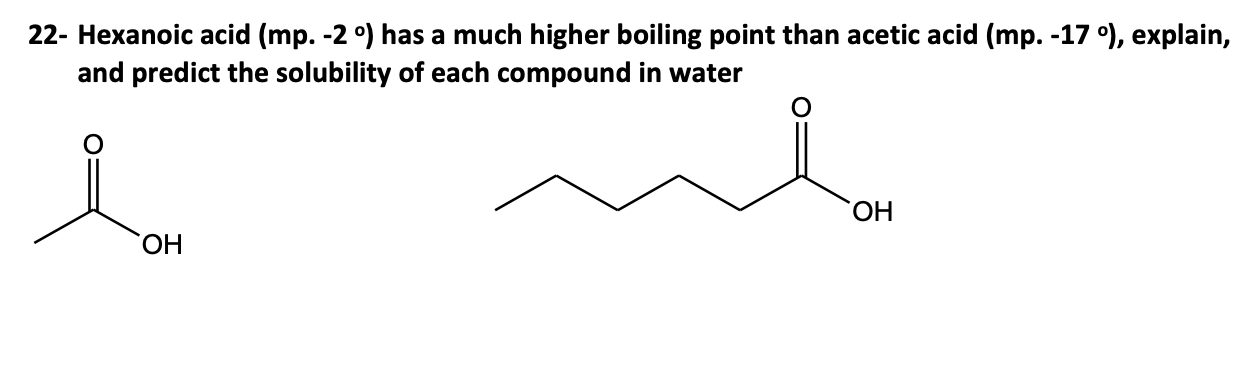
"Hexanoic acid (mp. -2 o) has a much higher boiling point than acetic acid (mp. -17 o), explain, and predict the solubility of each compound in water" i know hexanoic acid is insoluble in water bc of the number of carbons is more than 5 and acetic acid is soluble. i just need to know why hexanoic acid has higher boiling point than acetic acid pls...
Still Need Help?
Join the QuestionCove community and study together with friends!
for reference...
Hexanoic acid has a higher boiling point because of the longer carbon chain. Those extra bonds give the molecule a more complex structure. The hydrogen bonding in hexanoic acid is more extensive and stronger compared to acetic acid, making it more difficult to break these bonds and, consequently, increasing its boiling point.
Can't find your answer?
Make a FREE account and ask your own questions, OR help others and earn volunteer hours!
Join our real-time social learning platform and learn together with your friends!
Join our real-time social learning platform and learn together with your friends!
Latest Questions
 Countless7Echos:
uuh I've been practicing just writing like poems and stuff uhh how did I do for these two? "It was already too late for the messenger already began to rot,
Countless7Echos:
uuh I've been practicing just writing like poems and stuff uhh how did I do for these two? "It was already too late for the messenger already began to rot,
 CloverKris:
The Mountain's Light ______________________ A moonlit field with fresh green grass and spotted neatly with the most unusual trees of blue leaves lies before
CloverKris:
The Mountain's Light ______________________ A moonlit field with fresh green grass and spotted neatly with the most unusual trees of blue leaves lies before
 CloverKris:
The Life of an Aware ______________________ Looking through my eyes I'm probably
CloverKris:
The Life of an Aware ______________________ Looking through my eyes I'm probably
 MAGABACK:
How good is this story? It's supposed to be with the theme of Edgar Allen Poe. I'd take suggestions if you have any.
MAGABACK:
How good is this story? It's supposed to be with the theme of Edgar Allen Poe. I'd take suggestions if you have any.
1 day ago
1 Reply
0 Medals
1 day ago
7 Replies
0 Medals
2 days ago
1 Reply
0 Medals
50 minutes ago
17 Replies
5 Medals