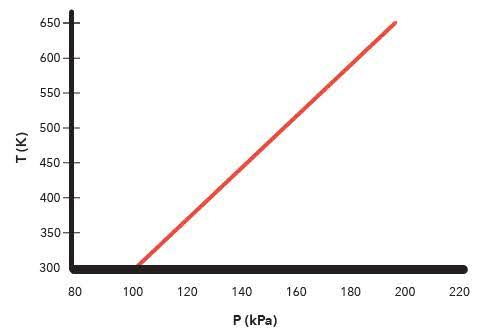
The graph below describes the temperature and pressure relationship of a gas under ideal conditions. Write 2 – 3 sentences to explain the graph. Be sure to explain what will happen to the real gas behavior as the temperature of the gas is lowered. That's the question and tbh I have no idea how this is related and this is due today womp womp does someone have chegg or like really know chem?
Well, what do you notice happens as the pressure P(kPa) increases?
i dont know this
as you can see in this image its your y=650 and you x=200 the point (200, 650) suggests that at a temperature of 200, the pressure of the gas is approximately 650,However, based on the ideal gas law (PV = nRT), we could infer that the pressure of the gas increases with increasing temperature, and that at a temperature of 200, the gas is at a high pressure. as the temperature is lowered, the gas behavior will deviate more significantly from the ideal gas law, and the pressure will deviate from the expected decrease, often resulting in a more dramatic decrease in pressure than predicted. tho it seems like you're describing a point on a graph, rather than the graph itself.hmm not sure why i went into science but hey ig its based on it
._.
Join our real-time social learning platform and learn together with your friends!