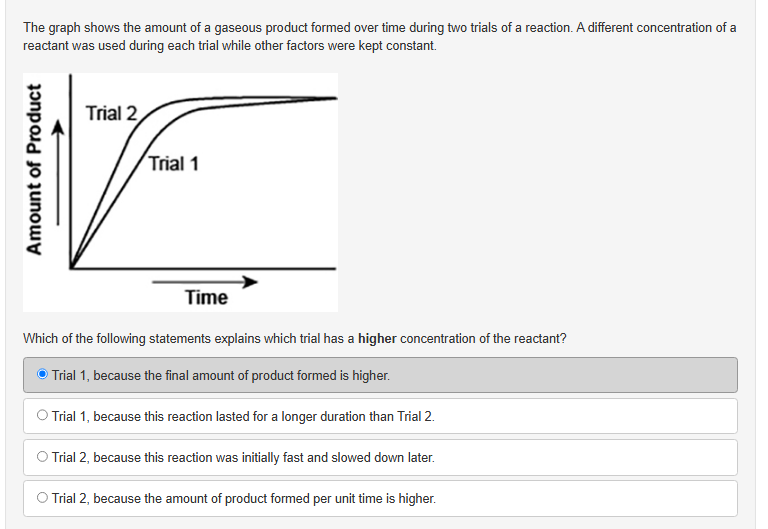
chem help
am i right with D here?
Yep because the question is asking for the trial with the higher concentration of reactants. A is wrong because the final amount of product formed is the same for both trials B is wrong because lasting longer indicates the reaction took a longer time to proceed, meaning it's a lower rate of reaction, remember the higher the concentration of the reactants, the faster the rate of reaction C is wrong because that doesn't compare or explain why the concentration of reactants is higher, just describes the graph D. Is correct because it explains that the amount of product formed per unit time is higher, and as we know rate of reaction is directly proportional to concentration of reactants
goat thank you so mch
understand it perfectly
Join our real-time social learning platform and learn together with your friends!