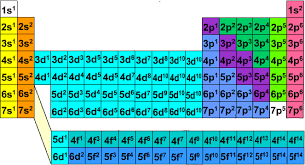
(ELECTRON CONFIGURATION) Okay so I tried finding the electron configuration for Iron (Fe / 26) and I got 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 and 4d^6 and apparently 4d^6 is wrong and its actually 3d^6 Can someone explain to me why its 3d^6 and not 4d^6 because I thought they were counted by how many rows it goes down. Thanks
The electron configuration for iron (Fe) is \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}3d^{6}\), not \(4d^{6}\), because electrons fill orbitals in order of increasing energy, not just the row number. The Aufbau principle dictates this order, and the \(4s\) orbital is lower in energy than the \(3d\) orbital, so it fills first. Therefore, after the \(3p\) shell is filled, the next two electrons go into the \(4s\) orbital before any fill the \(3d\) orbital
well the 4s orbital is filled before the 3d orbital
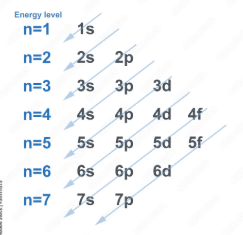
use this chart, it shud help u a lot more
Join our real-time social learning platform and learn together with your friends!