AP chem question PLEASE HELP MY GOSH how would I go about doing this?
usually I'm pretty good in chem but I don't even know how to do that... I wish I could help you with that, but I cant
thx anyways man
By paying attention to your classes so you can comprehend the material...
yo unhelpful bro TuT I got an A last semester (also it's asynchronous XD, no class to pay attention to)
still have an A, just struggling with one question, I'll figure it out
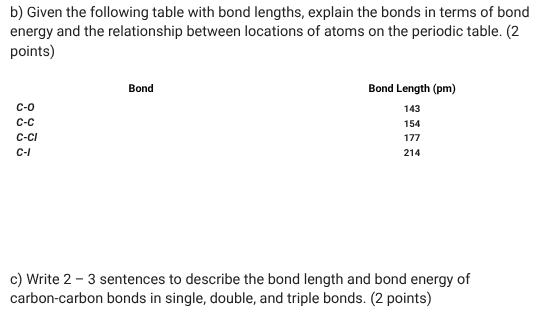
Shorter bonds typically have higher energy because the energy and distance are inversely related. In carbon-carbon bonds, bond length decreases and bond energy increases as bond order increases. A single bond would be the weakest and the longest bond. Remember triple bonds involve sharing of several electrons, making it tighter and therefore stronger.
When regarding the question focus on those details.
you can talk about, as bond length increases bond energy decreases, because size of atom decreases going across the period but increases going down the group
Join our real-time social learning platform and learn together with your friends!